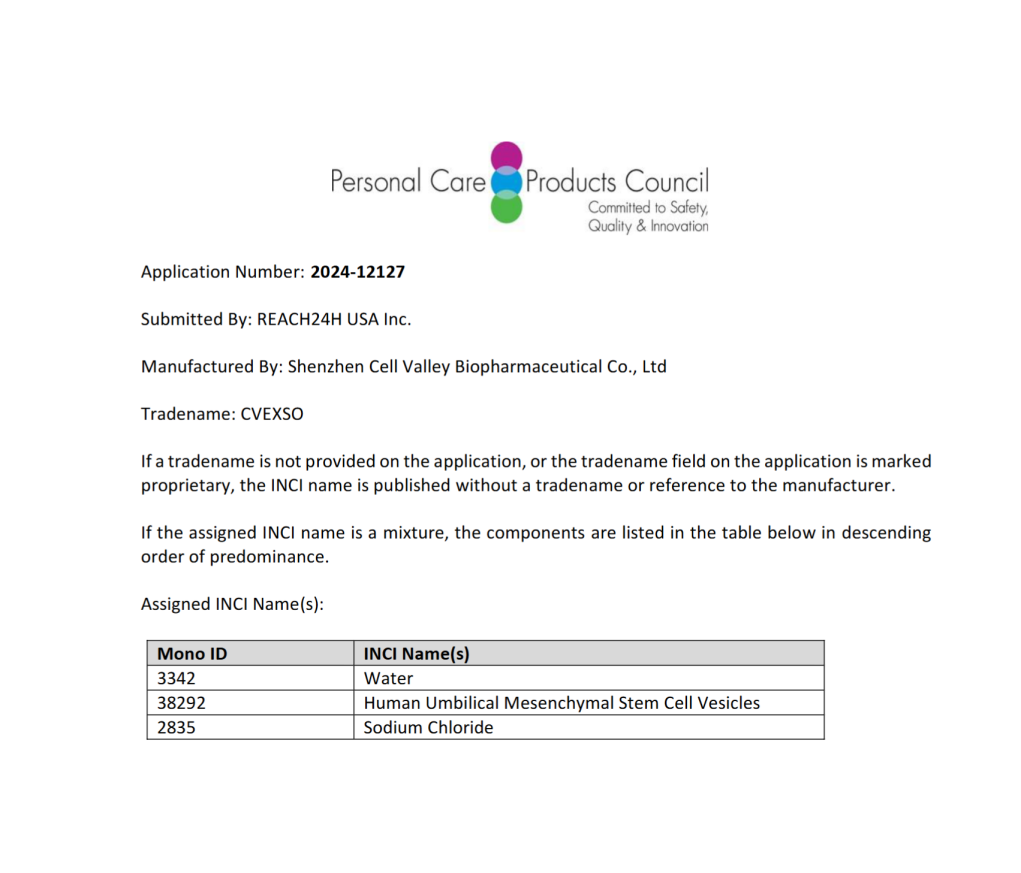
Good news | Shenzhen Cell Valley Exosomes have been certified by INCI, opening a new era of active ingredients in cosmetics
Recently, Shenzhen Cell Valley Biopharmaceutical Co., Ltd. (hereinafter referred to as "Shenzhen Cell Valley") has successfully obtained certification from the International Cosmetic Ingredient Naming Committee (INC) for its independently developed "Umbilical Mesenchymal Stem Cell Vesicles" (INCI code: 38292). The product, commercially named CVEXSOTM, has been officially included in the *International Cosmetic Ingredient Dictionary and Handbook*. This milestone signifies a breakthrough in international standards for bioactive cosmetic ingredients by Chinese enterprises, injecting revolutionary technological power into the global anti-aging skincare market.
 Umbilical cord mesenchymal stem cell vesicles are excellent carriers for the "cell-free therapy" strategy
Umbilical cord mesenchymal stem cell vesicles are excellent carriers for the "cell-free therapy" strategy
Umbilical cord mesenchymal stem cell vesicles (UCMSC vesicles) serve as the primary substance in stem cell paracrine action and are among the most extensively studied and applied exosomes. These vesicles contain bioactive substances such as therapeutic growth factors and mRNA similar to stem cells, demonstrating therapeutic effects including tissue repair and immune regulation. They also possess unique advantages like non-self-replicating properties and tumor-protective characteristics. With nanoscale dimensions that prevent capillary blockage, they can penetrate the blood-brain barrier to reach central nervous system injury sites for biological intervention. As a novel "cell-free therapy" strategy, UCMSC vesicles exhibit therapeutic benefits including immune modulation, anti-inflammatory effects, anti-fibrotic properties, oxidative stress suppression, and enhanced angiogenesis.
INCI certification: the "international passport" for Chinese ingredients to go global
The INCI name serves as the exclusive global identifier for cosmetic ingredients in R&D, production, and trade. After two years of technical validation and regulatory approval, Shenzhen Cell Valley's CVEXSOM has achieved certification that signifies: 1) Technical Authority: This ingredient can be legally used in global cosmetic formulations as an internationally recognized "stem cell vesicle" active ingredient; 2) Industry Standardization: Its name, manufacturing process, and quality specifications have been incorporated into the wINCI database, establishing traceable technical standards for the industry; 3) Market Access Advantages: Clearing regulatory hurdles for downstream brands to enter international markets like Europe, America, and Southeast Asia, thereby accelerating product launch cycles. CVEXSOTM: A "cell-level solution" that rewrites the anti-aging track
CVEXSOTM: A "cell-level solution" that rewrites the anti-aging track
CVEXSOTM utilizes Shenzhen Cell Valley's proprietary "Stem Cell Vesicle Directed Enrichment Technology" to overcome limitations in exosome activity. Key advantages include: 1) Exceeding 30 billion exosomes per vial, ensuring richer active ingredient delivery. 2) Pioneering high-concentration raw materials: The company produces world-leading active ingredients with concentrations reaching 650 billion cells. 3) Enhanced uniformity: Vessels maintain precise particle diameters of 55-60 nanometers, significantly improving absorption efficiency. 4) Rigorous quality control: Certified by two independent third-party testing agencies, the products meet stringent standards for superior quality and safety.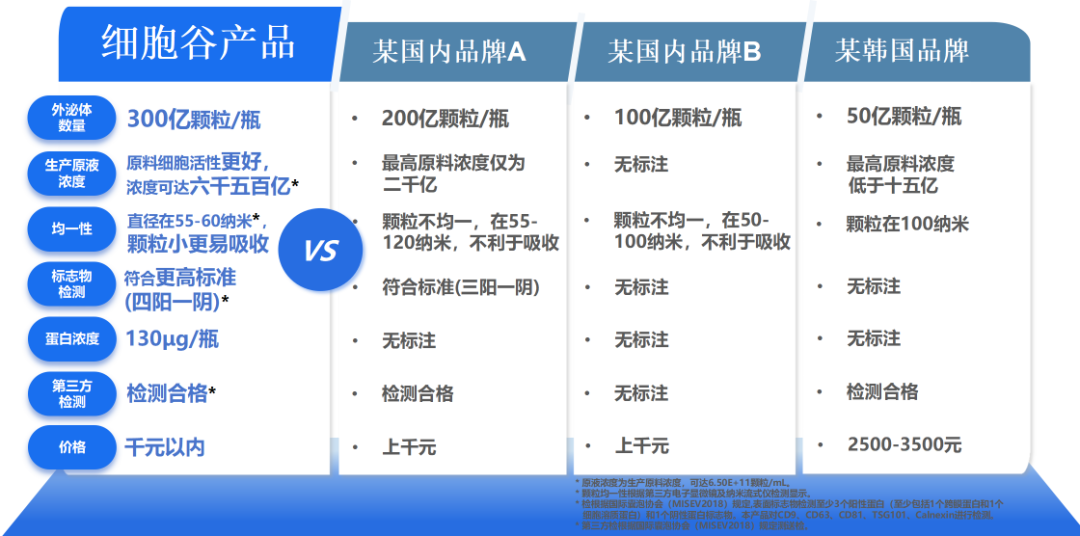
Umbilical cord mesenchymal stem cell vesicles (UCMSC vesicles) serve as the primary substance in stem cell paracrine action and are among the most extensively studied and applied exosomes. These vesicles contain bioactive substances such as therapeutic growth factors and mRNA similar to stem cells, demonstrating therapeutic effects including tissue repair and immune regulation. They also possess unique advantages like non-self-replicating properties and tumor-protective characteristics. With nanoscale dimensions that prevent capillary blockage, they can penetrate the blood-brain barrier to reach central nervous system injury sites for biological intervention. As a novel "cell-free therapy" strategy, UCMSC vesicles exhibit therapeutic benefits including immune modulation, anti-inflammatory effects, anti-fibrotic properties, oxidative stress suppression, and enhanced angiogenesis.
INCI certification: the "international passport" for Chinese ingredients to go global
The INCI name serves as the exclusive global identifier for cosmetic ingredients in R&D, production, and trade. After two years of technical validation and regulatory approval, Shenzhen Cell Valley's CVEXSOM has achieved certification that signifies: 1) Technical Authority: This ingredient can be legally used in global cosmetic formulations as an internationally recognized "stem cell vesicle" active ingredient; 2) Industry Standardization: Its name, manufacturing process, and quality specifications have been incorporated into the wINCI database, establishing traceable technical standards for the industry; 3) Market Access Advantages: Clearing regulatory hurdles for downstream brands to enter international markets like Europe, America, and Southeast Asia, thereby accelerating product launch cycles.
CVEXSOTM utilizes Shenzhen Cell Valley's proprietary "Stem Cell Vesicle Directed Enrichment Technology" to overcome limitations in exosome activity. Key advantages include: 1) Exceeding 30 billion exosomes per vial, ensuring richer active ingredient delivery. 2) Pioneering high-concentration raw materials: The company produces world-leading active ingredients with concentrations reaching 650 billion cells. 3) Enhanced uniformity: Vessels maintain precise particle diameters of 55-60 nanometers, significantly improving absorption efficiency. 4) Rigorous quality control: Certified by two independent third-party testing agencies, the products meet stringent standards for superior quality and safety.



